
Sam Lombardo, P.Eng., GMP Engineering
Timothy Leung, P.Eng., GMP Engineering
Tabi Salimi, GMP Engineering
Michael Mellor, SME, GMP Engineering
Ali Soeherman, P.Eng., GMP Engineering
Antibody Drug Conjugates (ADCs) are among the most potent targeted therapies in oncology, combining the selectivity of monoclonal antibodies with the cytotoxic strength of chemotherapeutics. Designing a manufacturing facility for ADCs requires a tightly integrated engineering approach that spans development, pilot, and GMP clinical manufacturing scales. This case study outlines a strategic framework for building scalable, flexible, and contained infrastructure capable of safely and efficiently
producing ADCs.
Antibody Drug Conjugates (ADCs) are among the most potent targeted therapies in oncology, combining the selectivity of monoclonal antibodies (mabs) with the cytotoxic strength of chemotherapeutics. Designing a manufacturing facility for ADCs requires a tightly integrated engineering approach that spans development, pilot, and GMP clinical manufacturing scales. This case study outlines a strategic framework for building scalable, flexible, and contained infrastructure capable of safely and efficiently
producing ADCs.
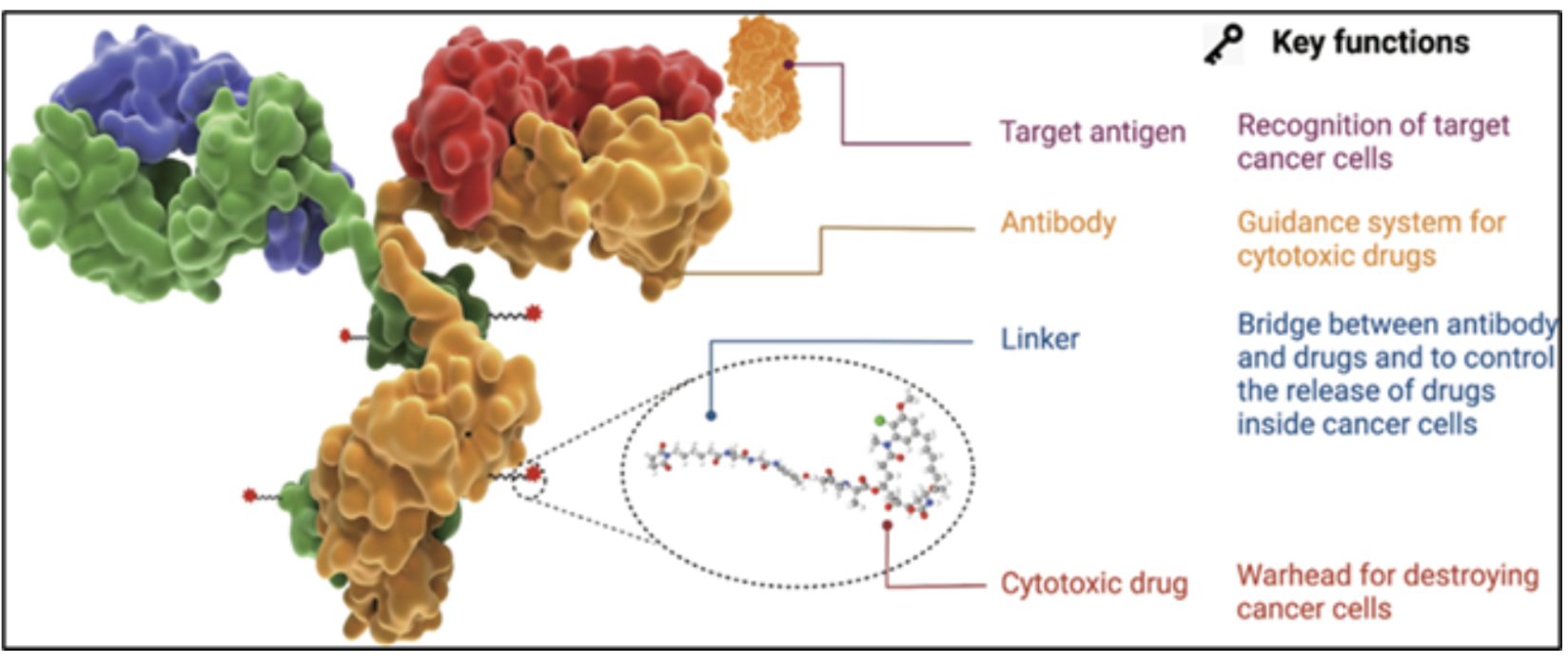
A typical ADC consists of three elements:
This design allows selective delivery of potent agents to tumor cells while minimizing off-target toxicity. However, the potency of the payload—often active at nanogram levels—requires stringent handling controls. Occupational Exposure Limits (OELs) are often <1 µg/m³, with payload handling being <10ng/m³ for the duration of the operation task. This profile drives the need for specialized containment and risk mitigation at every step of manufacturing. Importantly, the required containment performance can vary depending on the process phase—such as handling the pure payload in powder form versus when it is conjugated with mABs—necessitating a review and confirmation of equipment suitability at each stage.
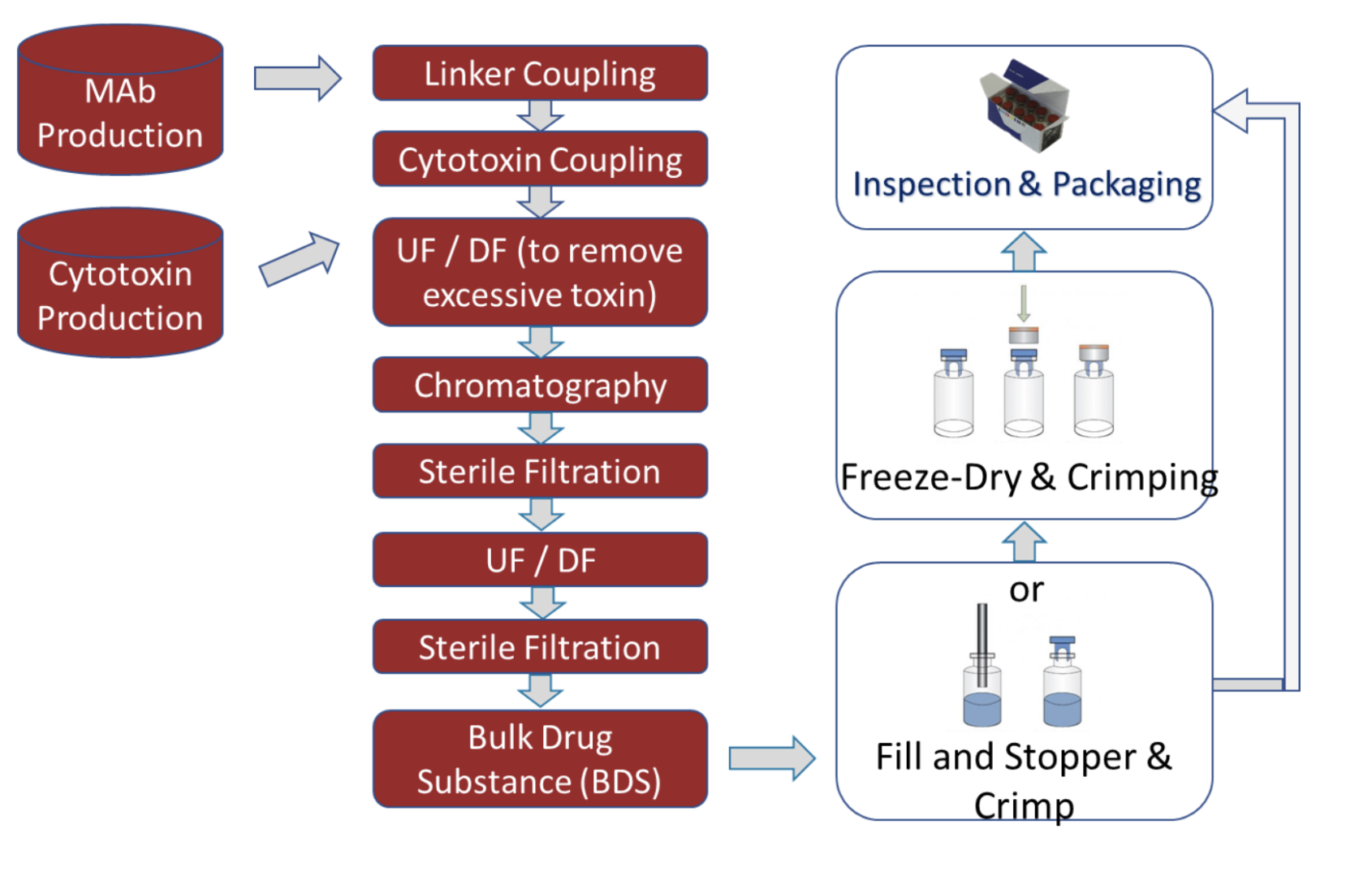
ADC production includes both Drug Substance (DS) and Drug Product (DP) workflows:
Each process step varies in terms of exposure risk (low to high) and influences facility design and containment strategy.
ADC facilities must accommodate varying batch sizes and process intensities as products progress through development.
Supporting Early-Stage ADC Process and Containment Design
Non-GMP Environment
Objective: Early conjugation chemistry optimization, formulation studies, and Drug-to-Antibody Ratio (DAR) characterization.
Emphasis: Exploratory testing, parameter screening, and process proof-of-concept under controlled containment.
Engineering Intent: Establish containment strategy, define process flow, and generate design data to support scale transition to pilot and kilo labs.

Bridging Process Development to Pilot Scale
Non-GMP Environment
Objective: Bridge PD to pilot scale; evaluate mixing, filtration, and transfer performance under contained conditions.
Emphasis: Establish process robustness, define operating ranges, and support early technology transfer.
Engineering Intent: Validate scale-up assumptions, confirm equipment design parameters, and guide the transition to GMP pilot or kilo labs.

Bridging Development to Clinical Manufacturing
GMP-Like or GMP Batches
Objective Support toxicology and early clinical studies; validate process scalability, containment performance, and automation strategy.
Emphasis: Process qualification, containment verification, and operator training under GMP-simulated conditions.
Engineering Intent: Confirm facility readiness, validate process control sequences, and generate data for full GMP clinical manufacturing transition.

Translating Process Design into Full GMP Manufacturing
cGMP Clinical & Commercial Operations
Objective: Support Clinical Phases I–III, process validation, and commercial launch manufacturing.
Emphasis: Full GMP compliance, automation, and implementation of advanced Process Analytical Technology (PAT).
Engineering Intent: Deliver robust, fully contained, and automated manufacturing environments that ensure safety, reproducibility, and regulatory compliance from clinical to commercial scale.
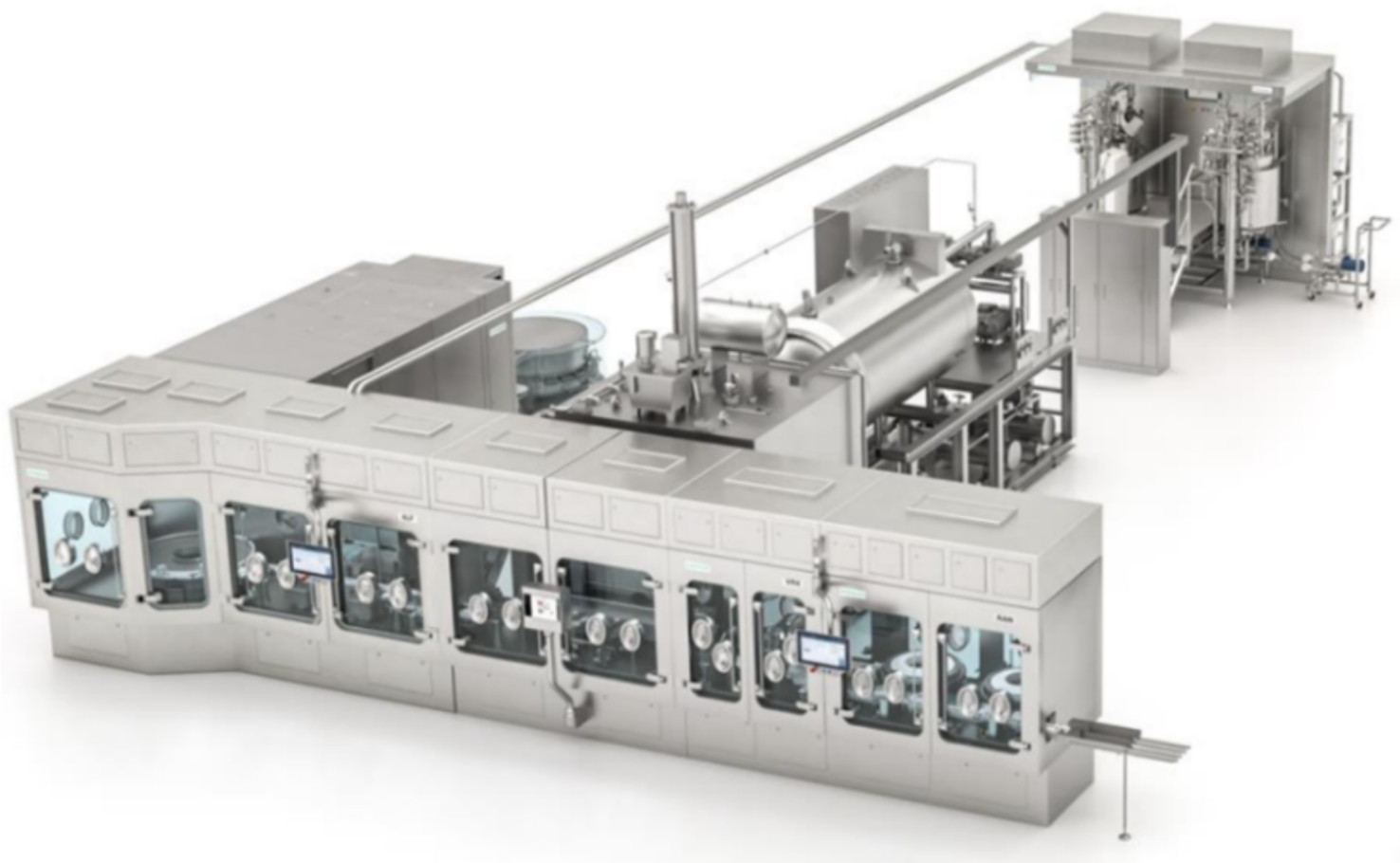
Single-use (SU) systems are favored throughout development and early clinical stages to reduce cleaning burden, lower cross-contamination risk, and accelerate changeovers. At larger scales, where throughput or process needs exceed SU capabilities, Wash-in-Place (WIP), Clean-In-Place (CIP) or Steam-in-Place (SIP) enabled stainless-steel systems are introduced. All systems are designed to facilitate closed processing wherever possible.
Drug Product operations—typically lyophilized sterile vials—are manual or semi-automated at small scale, transitioning to integrated fill/finish isolators at larger scale. Post-fill vial cleaning / decontamination ensures safe downstream handling, often performed using external vial washers or isolator-integrated decontamination systems (e.g., WFI spray, H₂O₂ vapor, or alcohol-based cleaning) to remove any potent drug residues from vial exteriors before inspection, labeling, and packaging