Sam Lombardo, P.Eng., GMP Engineering
Timothy Leung, P.Eng., GMP Engineering
Michael Mellor, SME, GMP Engineering
Ali Soeherman, P.Eng., GMP Engineering
Antibody Drug Conjugates (ADCs) are among the most potent targeted therapies in oncology, combining the selectivity of monoclonal antibodies with the cytotoxic strength of chemotherapeutics. Designing a manufacturing facility for ADCs requires a tightly integrated engineering approach that spans development, pilot, and GMP clinical manufacturing scales. This case study outlines a strategic framework for building scalable, flexible, and contained infrastructure capable of safely and efficiently
producing ADCs.
Antibody Drug Conjugates (ADCs) are among the most potent targeted therapies in oncology, combining the selectivity of monoclonal antibodies (mabs) with the cytotoxic strength of chemotherapeutics. Designing a manufacturing facility for ADCs requires a tightly integrated engineering approach that spans development, pilot, and GMP clinical manufacturing scales. This case study outlines a strategic framework for building scalable, flexible, and contained infrastructure capable of safely and efficiently
producing ADCs.
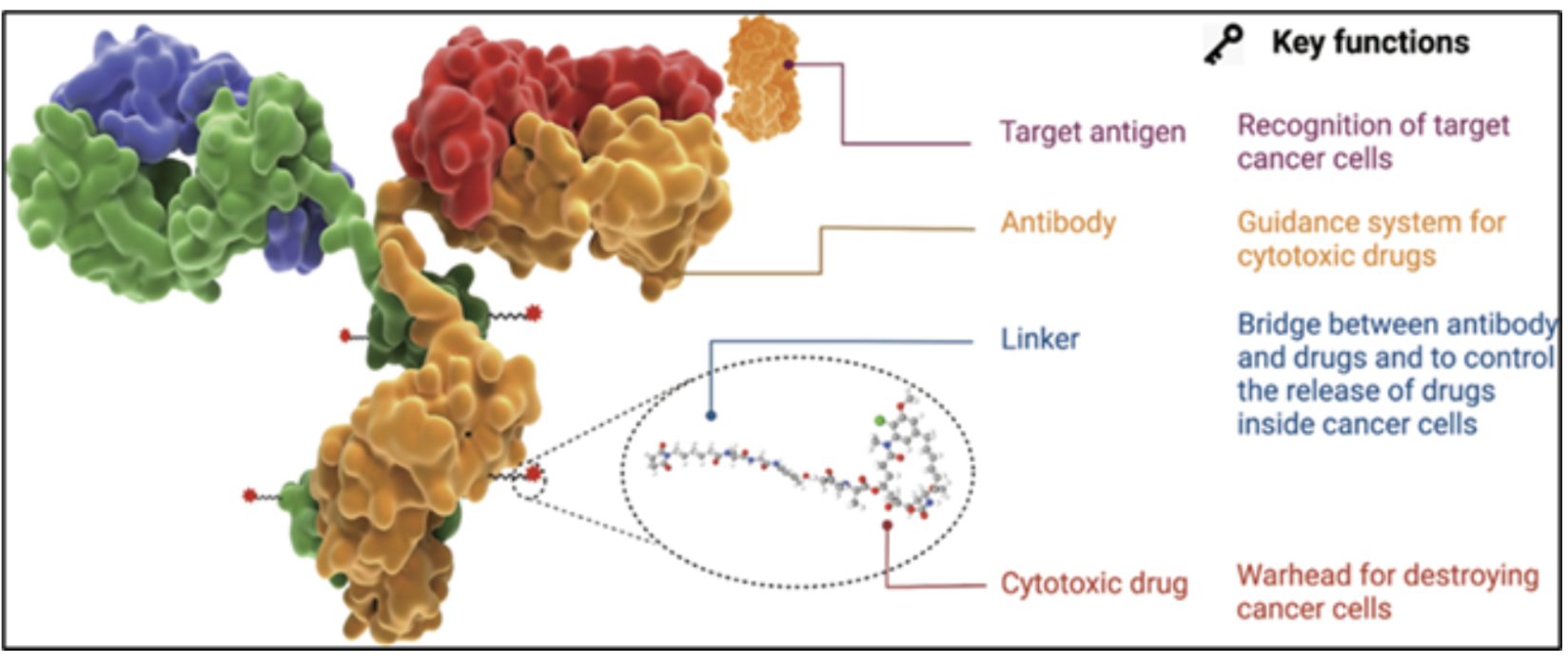
A typical ADC consists of three elements:
- A monoclonal antibody (mAb) that targets cancer cells
- A cytotoxic payload ("warhead")
- A chemical linker that joins them
This design allows selective delivery of potent agents to tumor cells while minimizing off-target toxicity. However, the potency of the payload—often active at nanogram levels—requires stringent handling controls. Occupational Exposure Limits (OELs) are often <1 µg/m³, with payload handling being <10ng/m³ for the duration of the operation task. This profile drives the need for specialized containment and risk mitigation at every step of manufacturing. Importantly, the required containment performance can vary depending on the process phase—such as handling the pure payload in powder form versus when it is conjugated with mABs—necessitating a review and confirmation of equipment suitability at each stage.